Health News Weekly Links 4/13/2025
A powerful new tool to prevent HIV; Shingles vaccine reduces dementia; STI epidemic in USA leveling off; New Biosecurity Forecasting Group
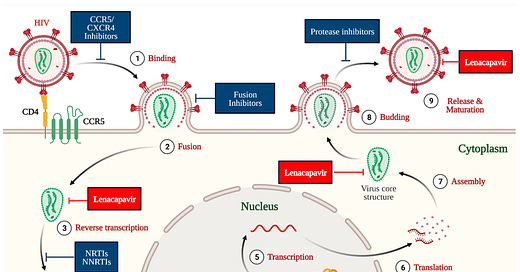
“As Gilead Sciences prepares to launch twice-yearly lenacapavir pre-exposure prophylaxis (PrEP) later this year, the company is also developing once-yearly formulations that could make an even greater contribution to ending the HIV epidemic.”
“A pair of phase III trials showed that twice-yearly lenacapavir is highly effective for HIV prevention. Results from the PURPOSE 1 trial showed that lenacapavir PrEP was 100% effective for young cisgender women in Africa – significantly more so than daily Truvada (tenofovir disoproxil fumarate / emtricitabine) pills. Findings from PURPOSE 2 showed that lenacapavir injections every six months reduced the risk of HIV acquisition by 96% relative to background incidence and by 89% compared to Truvada for gay and bisexual men and gender-diverse people in the United States and six other countries. In both trials, the injections were safe and generally well-tolerated.”
“Researchers reported the first phase I data on two longer-lasting [yearly] formulations [of lenacapavir]… at the Conference on Retroviruses and Opportunistic Infections (CROI 2025) and in The Lancet. The company considers the results promising enough to skip directly to a phase III trial.”
“Once-yearly intramuscular lenacapavir for PrEP has the potential to improve PrEP uptake and persistence and thus improve the scalability and public health impact of PrEP in populations who would benefit most,” Dr Renu Singh, Gilead’s director of clinical pharmacology, and colleagues concluded.”
“While these results are ground-breaking, researchers, advocates and global health officials stress that the promise of long-acting PrEP can only be realised if it is widely available and affordable to everyone who needs it worldwide. Gilead announced last year that it will work with pharmaceutical manufacturers to produce and sell generic lenacapavir for PrEP in more than 100 resource-limited countries with high HIV incidence.”
^Image by Dzinamarira, T.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M.; Aljuaid, A.; Alsharif, A.; Khan, A.; Kamal, M.; Rabaan, A.A.; Alfaraj, A.H.
A new study published in Nature finds that the shingles vaccine reduces dementia incidence by 20%!
This quasi-experimental regression discontinuity study provides causal evidence by exploiting a sharp discontinuity in the number of people eligible for and receiving the zoster (shingles) vaccine in Wales.
“we show that receiving the zoster vaccine reduced the probability of a new dementia diagnosis over a follow-up period of 7 years by 3.5 percentage points (95% confidence interval (CI) = 0.6–7.1, P = 0.019), corresponding to a 20.0% (95% CI = 6.5–33.4) relative reduction. This protective effect was stronger among women than men.”
“…with the caveat that these exploratory analyses are suggestive only, our analyses indicate that both a mechanism of action through a reduction in clinical and subclinical reactivations of VZV as well as through a VZV-independent immunomodulatory effect are plausible.”
The surge in STIs in the USA appears to have stopped, and partially reversed.
“The number of STIs decreased 1.8% from 2022 to 2023, reflecting decreases in gonorrhea (7.2% decrease), stable trends in chlamydia (<1.0% change), and an increase in total syphilis (all stages and congenital syphilis combined) (1.0% increase).”
The NYT suggests some possible reasons why, though they don’t provide causal evidence:
“The drop in cases may be the result of a variety of factors: an infusion of funds into health departments during the Covid-19 pandemic, changes in sexual behavior among gay and bisexual men because of the mpox outbreak in 2022, and the recent availability of the antibiotic doxycycline to forestall S.T.I.s after unprotected sexual encounters.”
There is a new expert Biosecurity Forecasting Group making public forecasts on the spread and impact of viral outbreaks, long-term trends in biosecurity innovation, the intersection of AI and biosecurity, potential policy changes, and other relevant developments.